Project
Project
An ambitious innovation project funded by the European Union, whose main goal is to develop innovative, minimally invasive, and personalized treatment solutions for tendon injuries
The project
Innovating Tendon Care, Restoring Movement
TEN4CARE is an ambitious innovation project funded by the European Union, whose main goal is to develop innovative, minimally invasive, and personalized treatment solutions for tendon injuries.
The project focuses on addressing the high prevalence of tendon and ligament disorders. Each year, around 33 million musculoskeletal injuries are reported, approximately 50% of which involve tendon and ligament damage. Since these injuries have diverse etiologies, there is no one-size-fits-all solution. These disorders are significantly debilitating, associated with severe pain, motor impairment, and loss of self-sufficiency.
TEN4CARE represents a crucial opportunity for European materials and MedTech industries. We aim to promote rapid recovery from acute or chronic conditions, addressing the associated health complexities.
Regeneration
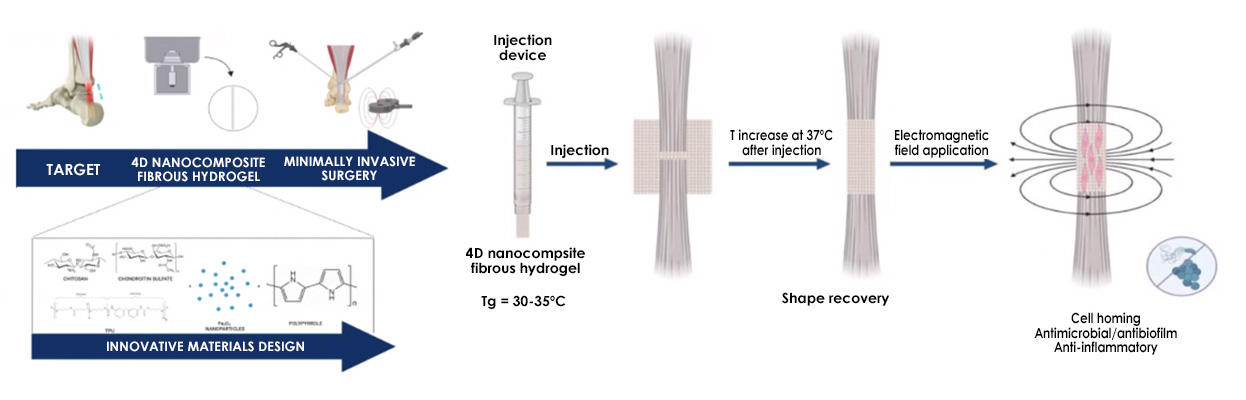
The main aim of the TEN4CARE project is to develop innovative, minimally invasive, and personalised treatment solutions for tendon injuries using advanced shape-memory biomaterials and 4D hydrogels. These novel biomaterials, designed to respond to external stimuli and loaded with an anti-inflammatory drug, will be doped with magnetic nanoparticles to significantly aid tendon repair, reduce recurrence rates, and promote tissue regeneration while minimising complications associated with current surgical approaches. 4D nanocomposite fibrous hydrogel doped with MNP and containing a polypyrrole network, both sensitive to electromagnetic field (EMF), will be manufactured using centrifugal spinning.
The fibers will be yarned to form a shape memory device able to be implanted as a rolled 3D structure using a cannula device and to envelop the tendon forming a cage to support tendon repairing process, thanks to the shape recovery at body temperature. The mechanical forces all acting within the pN range and the mV electric stimulation, given by the application of EMF after implant, will trigger the mechanotransduction and interfere with the quorum sensing both enhancing ECM production and avoiding microbial colonisation/infection.
By integrating digital modelling and advanced manufacturing techniques, with a focus on industrial and societal sustainability, TEN4CARE seeks to address the growing clinical and economic burden of tendon disorders with more effective, scalable, and sustainable treatment options.
The main aim of the TEN4CARE project is to develop innovative, minimally invasive, and personalised treatment solutions for tendon injuries using advanced shape-memory biomaterials and 4D hydrogels. These novel biomaterials, designed to respond to external stimuli and loaded with an anti-inflammatory drug, will be doped with magnetic nanoparticles to significantly aid tendon repair, reduce recurrence rates, and promote tissue regeneration while minimising complications associated with current surgical approaches. 4D nanocomposite fibrous hydrogel doped with MNP and containing a polypyrrole network, both sensitive to electromagnetic field (EMF), will be manufactured using centrifugal spinning.
The fibers will be yarned to form a shape memory device able to be implanted as a rolled 3D structure using a cannula device and to envelop the tendon forming a cage to support tendon repairing process, thanks to the shape recovery at body temperature. The mechanical forces all acting within the pN range and the mV electric stimulation, given by the application of EMF after implant, will trigger the mechanotransduction and interfere with the quorum sensing both enhancing ECM production and avoiding microbial colonisation/infection.
By integrating digital modelling and advanced manufacturing techniques, with a focus on industrial and societal sustainability, TEN4CARE seeks to address the growing clinical and economic burden of tendon disorders with more effective, scalable, and sustainable treatment options.
Methodology
A rigorous and holistic multi-disciplinary
The TEN4CARE methodology is a rigorous and holistic multi-disciplinary approach that integrates advanced biomaterial design, molecular simulations, and industrial engineering with exhaustive preclinical validation, all framed by the principles of Safe and Sustainable by Design (SSbD) and end-user engagement (PPIE).
The initial phase focuses on the design and development of the core innovation: an electromagnetic-responsive shape memory 4D nanocomposite fibrous hydrogel.
This begins with molecular dynamics simulations (using GROMACS) to predict the shape memory behaviour of the polyurethane (PU) structure, optimizing the actuation temperature to the physiological range of 32-35°C.
The innovative biomaterial will be synthesized by incorporating bioactive polysaccharides (chitosan and chondroitin sulphate) and peptidomimetic antimicrobial motifs (AMP) to enhance healing and prevent colonization.
A key technical step is the optimization of the in situ synthesis (in solid phase) of magnetic nanoparticles (MNPs) and polypyrrole within the polymer blend, using an eco-sustainable process designed for scalability.
The second phase concentrates on industrialization, 4D programming, and digital integration. The nanocomposite fibrous hydrogel scaffold is manufactured using centrifugal spinning technology, a sustainable and high-yield method. The resulting fibres are assembled into a yarn and undergo shape memory programming.
This process allows the scaffold to be rolled for implantation via minimally invasive surgery (MIS) equipment, facilitating its delivery and subsequent recovery into a predefined “cage structure” upon reaching body temperature to envelop the tendon. To ensure compliance with GMP (Good Manufacturing Practice) standards and promote production robustness, an automated centrifugal spinning equipment and a Digital Twin (software program) will be designed and developed.
This Digital Twin, assisted by an AI framework, simulates and optimizes the manufacturing process. Additionally, the hydrogel is loaded with an anti-inflammatory drug (NSAID or corticosteroid) to ensure its controlled release during the acute phase of inflammation and pain post-surgery, targeted for approximately 15 days.
The final phase involves stringent preclinical characterization and strategic translation. Rigorous validation includes characterizing the structural, magnetic, and mechanical properties (aiming for ultimate tensile strength 5–10 MPa) and verifying a slow degradation rate (expected not higher than 30% in 3 months, supporting healing for at least 6 weeks). In vitro tests assess mechanotransduction pathways (quantifying gene expression like YAP1 and Tenascin C) and antimicrobial/antibiofilm properties.
In vivo preclinical evaluation is performed using both a small animal model (rats) and a clinically relevant large animal model (sheep, for total Achilles tendon rupture) under GLP (Good Laboratory Practice) conditions. The entire development is guided by the SSbD framework, including quantitative assessments via LCA, LCC, and S-LCA to ensure safety and sustainability.
Final exploitation is secured through regulatory planning (MDR/EMA), economic evaluation via a Cost-Effectiveness Model, and user feedback integrated through PPIE methods. Furthermore, a feasibility study will investigate developing a Digital Twin software program for tendon lesion and scaffold geometry in view of a personalized approach.



